After swallowing a liquid, we always exhale, and during exhalation, retronasal olfaction occurs. Liquid reaches the pharyngeal wall and is then pushed upwards towards the olfactory epithelium as we exhale. It’s called ‘retronasal’ because aromatic gases enter the nasal cavity from the back of the nose instead of the front. When we aspirate (slurp), liquid is thrown to the back of the mouth and lands on the pharyngeal wall, just as it does during normal swallowing.
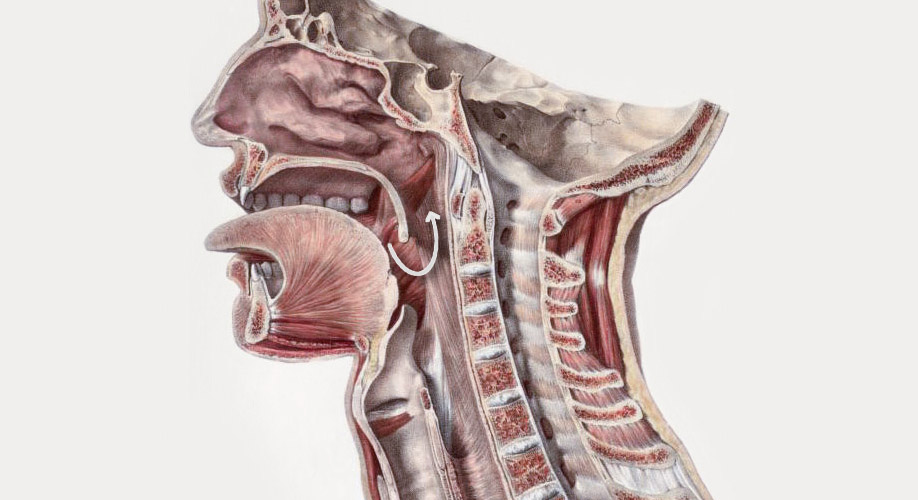 Illustration: Path from the pharyngeal wall to olfactory epithelium
Illustration: Path from the pharyngeal wall to olfactory epithelium
An added benefit of aspirating liquid as you sample coffee is that it delivers aromatic gases to your olfactory epithelium almost instantly. It is more efficient than waiting for aromatics to reach the olfactory epithelium through retronasal olfaction (H. B. Heath, 1988).
Here’s how to aspirate like World Barista Champion Gwilym Davies!
In order for humans to experience the flavour of a beverage, it is not enough for it to simply contain a high concentration of dissolved aromatic gases. These dissolved aromatics have change back into their gaseous states soon after we slurp them up; otherwise, the cilia extending from our olfactory sensory neurons won’t know that any aromas are present. To guarantee that a lot of the aromatic gases change from a liquid into a gas before we swallow or spit them out, we need to reduce the vapour pressure surrounding the liquid. A change in vapour pressure encourages the aromatics to leave the liquid more quickly then they are likely to be dissolved into it. This situation is known as non-equilibrium volatilisation.
The most effective way to achieve non-equilibrium volatilisation with a liquid is to nebulise it by drawing it into our mouths from a spoon in an aggressive slurping motion. We must be careful not to inhale any of the liquid as we aspirate. It can be helpful when learning how to aspirate, to lean your head slightly forward as you slurp. H. B. Heath (1988) explains that the act of nebulising a liquid through aspirating leads to an increase in the concentration of volatiles that escape a liquid compared with ordinary swallowing.