What’s the difference between temporary and permanent hardness and does it matter for coffee brewing?
Hardness is a measure of the amount of certain minerals in water. The amount and proportion of minerals in your brewing water can have a dramatic effect on the flavour of your coffee, as well as affect how likely your espresso machine is to get scaled up, so it’s worth trying to understand a few key concepts.
The term ‘hardness’ originally comes from the effect of minerals in water on soap. The minerals in hard water bind to soap and form an insoluble ‘scum’, making it harder to form a foam, and making the soap less effective in washing. At some point, people noticed that if the water was boiled before use, the water would become less hard, making washing easier. The hardness that could be removed by boiling is referred to as temporary hardness, the hardness that remains, no matter how much you boil the water, is called permanent hardness.
So the definition is easy enough to remember, but to really understand what’s going on, we need to know a little bit about what minerals in water are made of. Each mineral that dissolves in water is made up of ions, which are electrically charged particles. The solid mineral has equal amounts of positive and negative charge mixed together, so the charges cancel each other out. For example, table salt, sodium chloride, is made up of equal parts positively charged sodium (Na+) and negatively charged chloride ions (Cl–). When the mineral dissolves in water, the ions split apart (‘dissociate’) and spread out in the water.
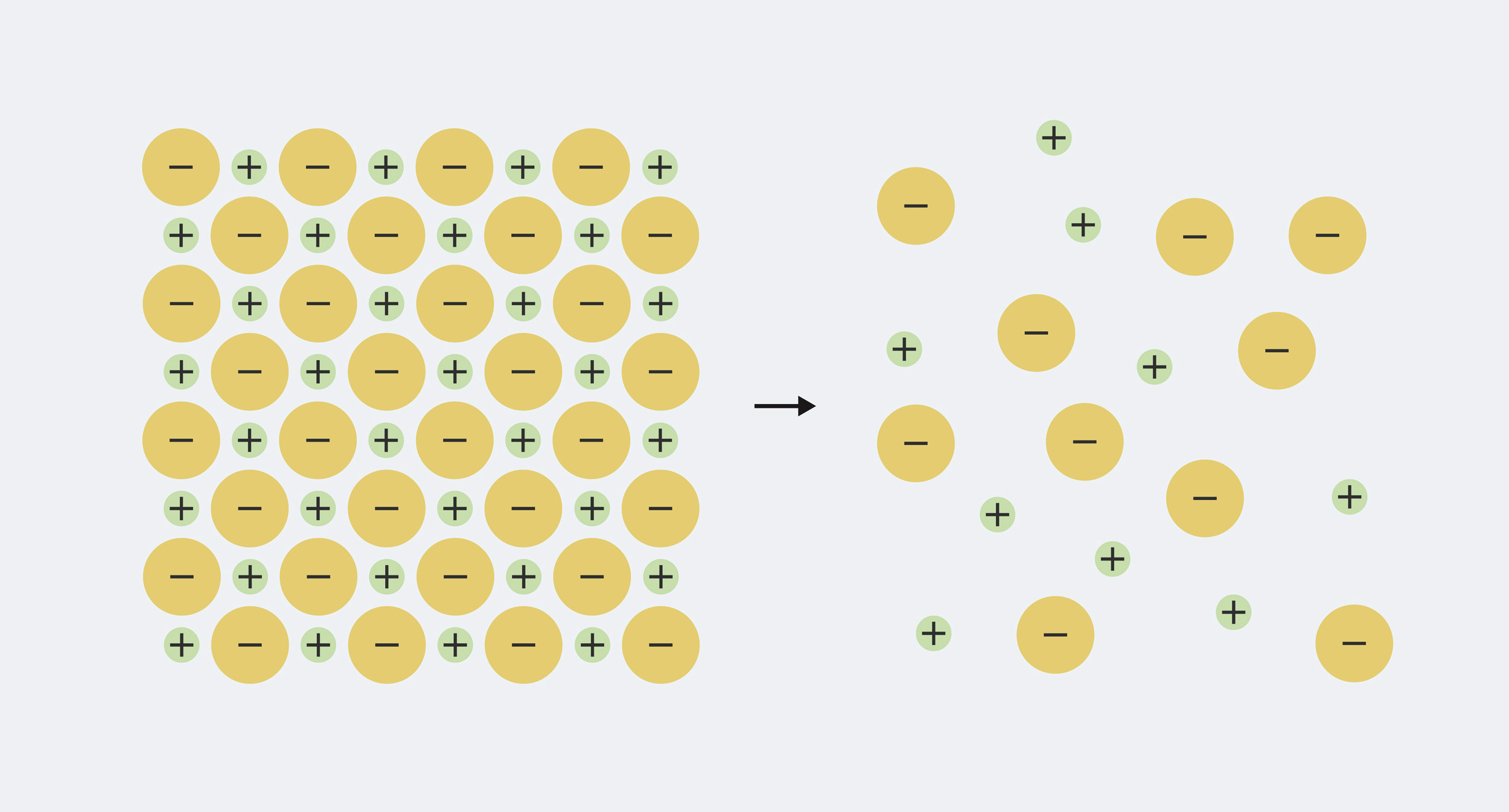 The sodium and chloride in solid table salt are packed tightly together. When it is dissolved in water, it separates into sodium ions (Na+) and chloride ions (Cl–)
The sodium and chloride in solid table salt are packed tightly together. When it is dissolved in water, it separates into sodium ions (Na+) and chloride ions (Cl–)
Hardness is caused by any mineral where the positively charged ion has more than one charge. The stronger charge in these ions is what causes it to interact with soap — and it is the same charge that means these minerals can help draw flavours out of coffee.
Calcium (Ca2+) and Magnesium (Mg2+) make up nearly all of the hardness in drinking water. Since calcium and magnesium both have two positive charges, they behave in a very similar way, so can be considered interchangeable when we talk about hardness. Some other ions, like iron (Fe3+) or aluminium (Al3+), can technically contribute (WHO 2011), but we normally ignore these, as the amount of them in drinking water should be very low.
So the total hardness, also called ‘general hardness’ or GH, is just a measure of the amount of positively charged calcium and magnesium that is in the water. Because the electrical charge in these ions helps to extract flavour molecules, the GH measurement is an indicator of the extraction power of the water.
Now remember that each positively charged mineral ion has to be paired with negatively charged ions as well. The most important of these in water is bicarbonate (HCO3–). When you boil water with bicarbonate in it, it reacts with any calcium and magnesium in the water to form a solid called limescale (calcium carbonate and magnesium carbonate). So this is what causes temporary hardness — by boiling the water, you are forming limescale, which drops to the bottom. The permanent hardness is whatever hardness is left over — any calcium or magnesium that doesn’t have any bicarbonate to react with.
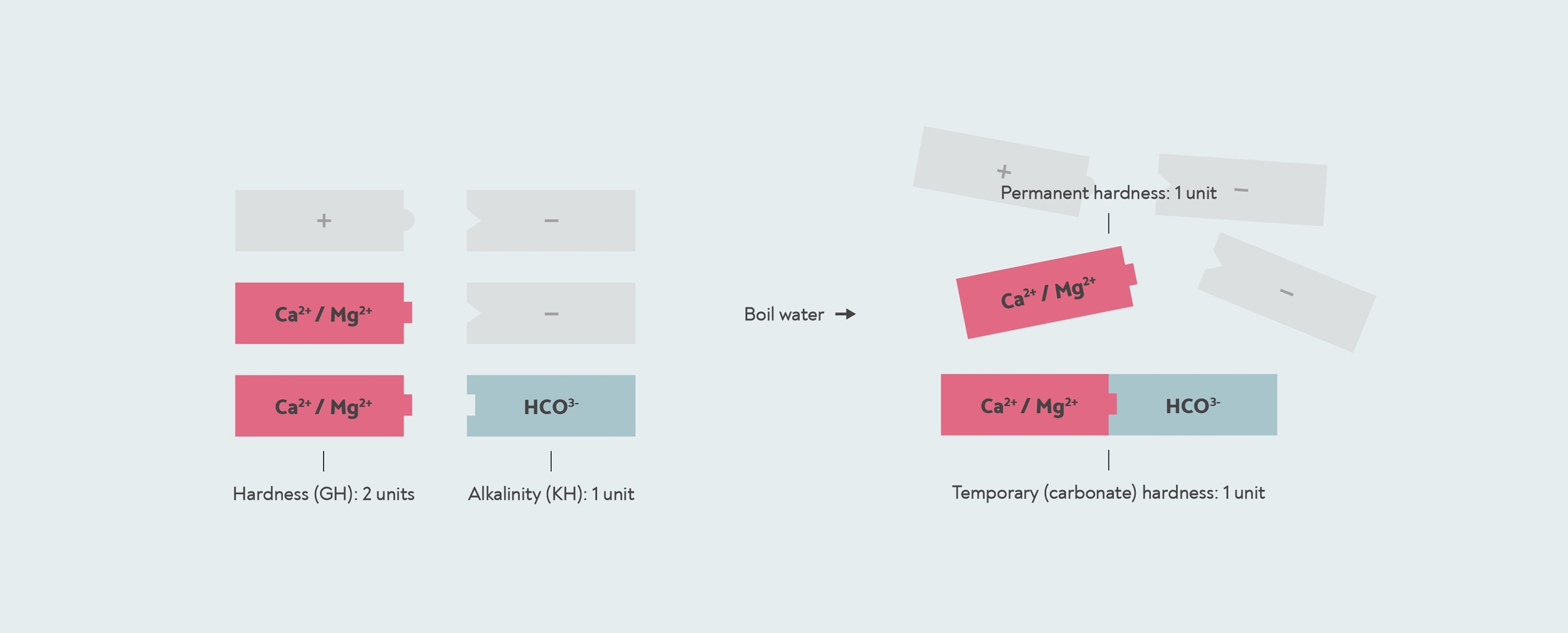 Temporary and permanent hardness. This water has a GH of 2, because there are two calcium/magnesium units (red) in it. It has a temporary hardness of 1, because one carbonate unit binds to one calcium/magnesium to form limescale. The other calcium/magnesium is permanent hardness that doesn’t form limescale and isn’t removed by boiling. The other positive and negative mineral ions in the water are shown in grey.
Temporary and permanent hardness. This water has a GH of 2, because there are two calcium/magnesium units (red) in it. It has a temporary hardness of 1, because one carbonate unit binds to one calcium/magnesium to form limescale. The other calcium/magnesium is permanent hardness that doesn’t form limescale and isn’t removed by boiling. The other positive and negative mineral ions in the water are shown in grey.
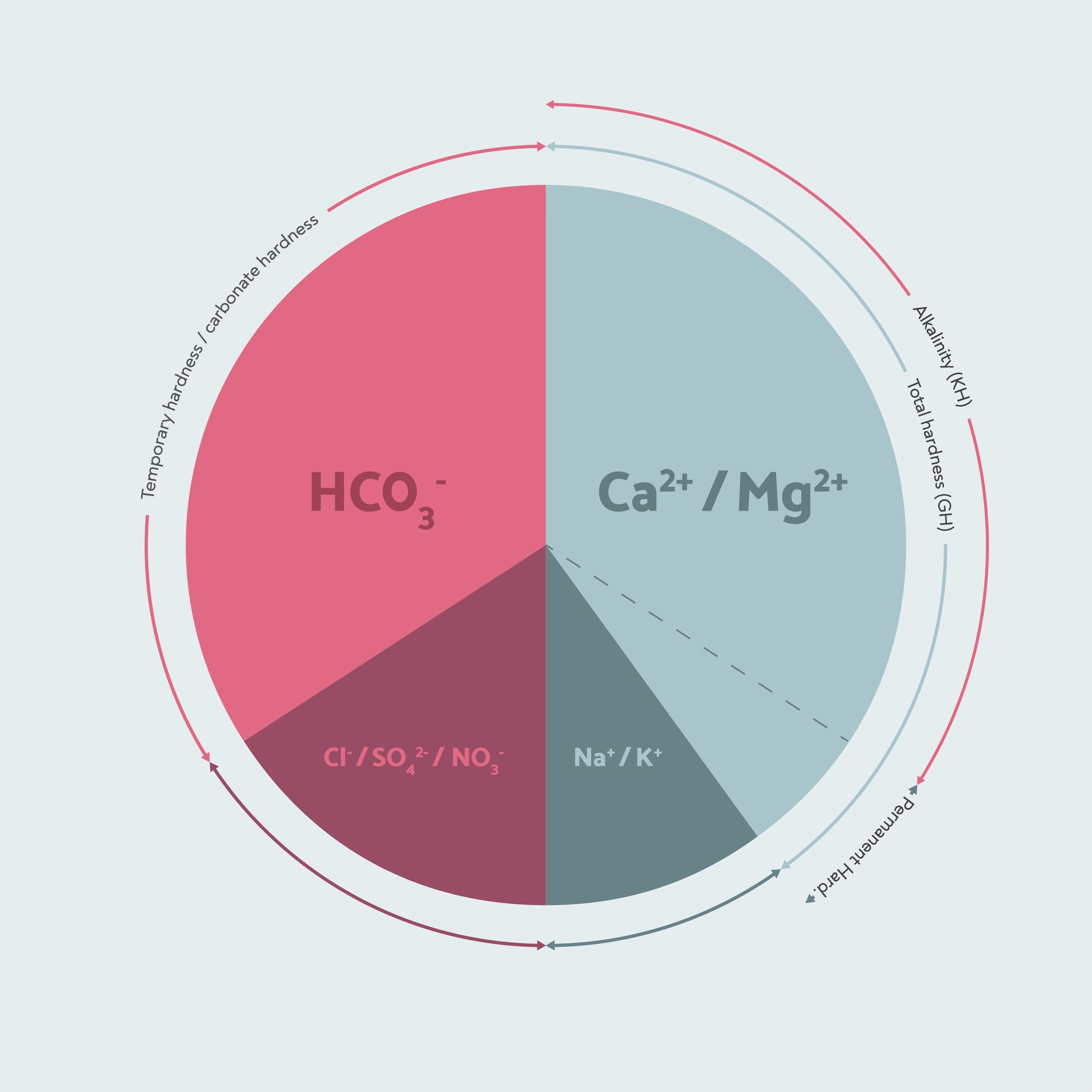
When we measure the hardness in our water, we usually make two measurements: GH and KH. GH is general hardness, and measures the total amount of calcium and magnesium in the water. KH stands for Karbonathärte, which is the German for Carbonate Hardness. Carbonate hardness is exactly the same as temporary hardness: it means all the hardness that is paired with bicarbonate ions, which can form limescale.
However, where it gets confusing is that a typical KH test doesn’t actually measure carbonate hardness. A KH drop test just measures the amount of bicarbonates in the water, also called the alkalinity. This measure is also important because the alkalinity buffers out acidity in the coffee, and makes it taste less sour. Too much alkalinity will make the coffee taste flat.
In our example so far, the alkalinity is the exact same as the temporary hardness, because there is enough calcium and magnesium to react with all of the bicarbonate and form limescale. This is the case in most natural drinking water — the alkalinity and the carbonate hardness or temporary hardness are the exact same, so most of the time the KH test does also tell you the carbonate hardness.
However, in some cases, the amount of bicarbonate can be more than the amount of calcium or magnesium. This can happen when the water has been softened, or when salt water has got into the drinking water. In this case, there might not be enough calcium and magnesium to react with all of the bicarbonate. With this kind of water, if you boil it, all the hardness is removed, so the temporary hardness is the same as the total hardness, and there is no permanent hardness at all.
In this case, the KH test will give you a higher result than your GH. When the result of a KH test is higher than the GH, then the carbonate hardness and the general hardness are actually the same. The number the KH test gives you is then actually the alkalinity, not the carbonate hardness.
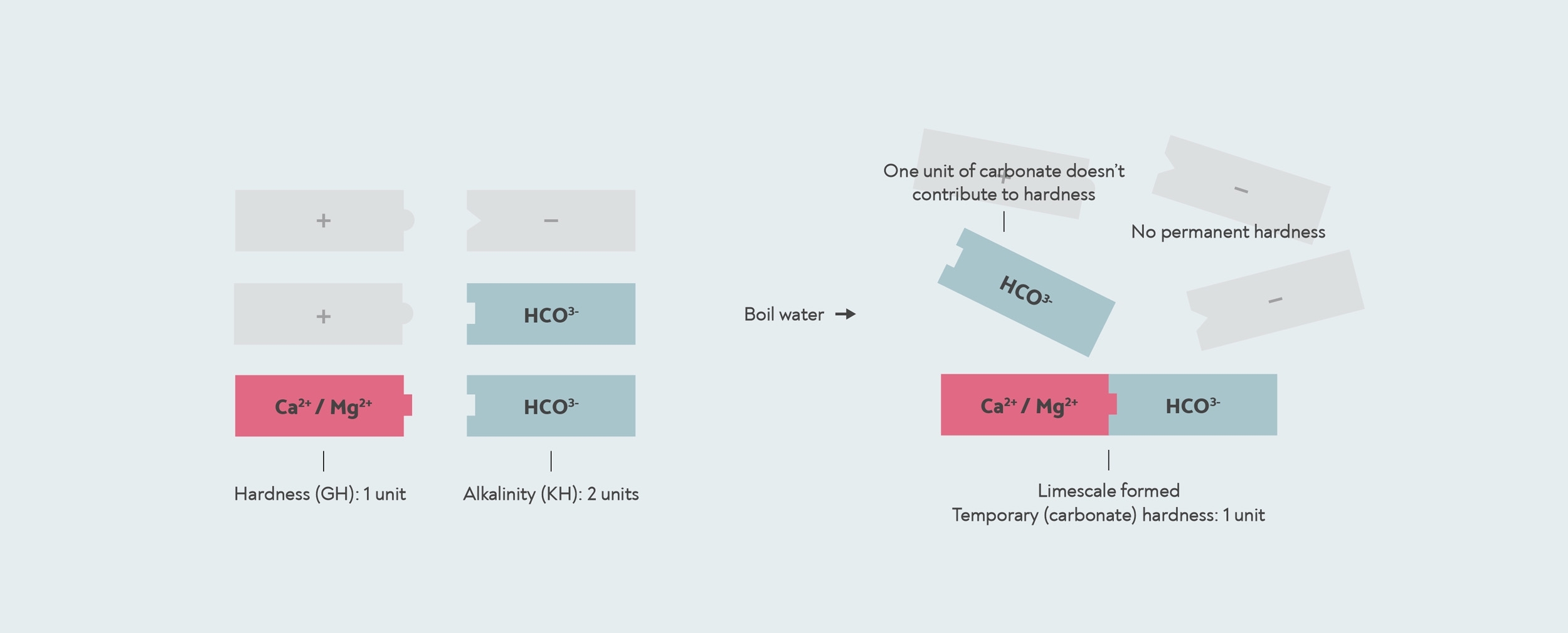 When the alkalinity is higher than the general hardness, then all the hardness is temporary or carbonate hardness. In this case, the remaining alkalinity does not contribute to hardness. The KH drop kit measures an alkalinity of 2, but the carbonate hardness is actually only 1, because there is only 1 unit of hardness for it to form limescale with.
When the alkalinity is higher than the general hardness, then all the hardness is temporary or carbonate hardness. In this case, the remaining alkalinity does not contribute to hardness. The KH drop kit measures an alkalinity of 2, but the carbonate hardness is actually only 1, because there is only 1 unit of hardness for it to form limescale with.
This case is rare enough in tap water, though, that most of us can ignore it when we’re testing our water. In most drinking water, KH = alkalinity = temporary hardness. Permanent hardness is just whatever hardness is left over, so: Permanent Hardness = GH − KH.
In summary, these are the three measures that really matter in coffee brewing: the total hardness or general hardness (GH), the temporary hardness, or carbonate hardness, and the alkalinity. The total hardness helps the water to extract flavour from the coffee. The temporary hardness is what determines how much of that hardness can form limescale. Finally, the alkalinity determines how much the water will remove acidity from the coffee.
If the KH is below GH, then the KH test gives you the figure for both the temporary hardness and the alkalinity, because KH determines how much of the GH can form limescale. If the KH is above GH though, then the KH just measures the alkalinity, and the temporary hardness is just the same as the total hardness, because the ‘extra’ KH can’t form limescale by itself.



Based on the explanation, I’m perplexed as to why there is this disclaimer on using the water recipes – “NB: If you are making your own water with magnesium salts (such as epsom salts) then the LSI calculation does not apply as no calcium is present. We do not recommend using this kind of water in espresso machines.” –
This page mentions that magnesium will bond with carbonates that are present, which results in KH (temporary hardness). This will be lightly scale forming and this is a good thing, as per one of the responses above, this creates a good cycle where a thin coating of protective limescale is formed on the boiler which continuously exchanges with the new remineralised water being added as the water boils and cools.
My objective is to create water that tastes great but is also safe to use in an espresso machine so can I use the recipes at this URL safely? https://www.baristahustle.com/app-archive-main/the-water-calculator/
Hi Samizzle. The thing is, if you create a coffee water using distilled water that does not contain any calcium in it, then even after adding your epsom salts and your bicarb to it via the concentrates we recommend here https://www.baristahustle.com/blog/diy-water-recipes-redux/, it is possible this water could still be non-scale forming and could also have the potential to rust out parts of your boiler or other components. That’s why we created the bottle calculator, to allow you to remineralisation a water that contains a known quantity of calcium to begin with. Of course you need to be careful you don’t end up with an overly scale forming water either so even if you find a nice base water that might have say 30milligrams/L of calcium in it according to the label on the bottle, be sure to test its GH too.
Interesting, I’m finding that just when I though I understood this, I understand nothing at all :S. I’m still trying to come to terms with the difference between magnesium and calcium in relation to limescale production given this statement, “Since calcium and magnesium both have two positive charges, they behave in a very similar way, so can be considered interchangeable when we talk about hardness.”
Are you saying that calcium carbonate deposits limescale more readily than magnesium carbonate? If so, is it possible to suggest what amount of calcium I could add into my 1L of hardness concentrate (in addition to the 2.45g Epsom salts that I’ve already added) to balance this properly and avoid corrosion?
That’s correct. Magnesium can form a kind of scale, but it doesn’t tend to stick to surfaces the same way as limescale (limescale is Calcium by definition).
The scale-forming potential of your water (as measured by LSI) depends on more than just the amount of Ca – it also depends on what else is present, and the pH of the water, and even the temperature. So I can’t give you a specific recommendation, but encourage you to check the LSI of your water with our Water Calculator.
Having said all of that, whether corrosion is a serious risk in your machine or not depends on what it is made of, and how it is built. Many machines run with distilled water with no problems for many years, others might see corrosion even with natural water. For a bit more information on this, check out the AMA we did all about water.
Excellent information, thank you. I have another question for you. Our water is relatively hard at 350-400 ppm. We are in the process of selecting either a water softener to pull out the mineral ions or a TAC water conditioner to force the ions to crystallize but does not remove them. How would the presence of these inert crystals affect the brew?
Hi Dan, thanks for commenting. We don’t have direct experience with TAC or related water treatment options (such as magnetic or electric water conditioners), but we are sceptical.
The way this type of water conditioner is said to work is to force microcrystals of calcium carbonate to form – in other words, tiny crystals of limescale. The theory is that by forming crystals in the water, you prevent it from forming on the metal surface of your water heater instead.
It’s possible that TAC can help reduce limescale forming like this in certain situations, but how well this works in most real-world settings is far from proven. This page has a good overview of what little is known about them, and points to a major flaw: crystals will only form if the water is supersaturated – so while it may work up to a point, it may not be as effective as a softener in getting to the hardness levels we like for coffee.
As they quote: “It can do nothing to prevent scale deposition at higher-temperature surfaces where the carbonates will be less soluble, nor can it remove the hardness ions down to a level at which they will not form scums with soap.” I suspect if the water is still hard enough to form a soap scum, it’s hard enough to have a negative effect on the coffee. It’s also worth bearing in mind that those crystals are not ‘inert’ – they are simply small crystals of limescale and there’s nothing to stop them being redissolved in the water.
If you – or any of our readers – try a TAC conditioner for their coffee, we would love to hear about your experience. But for now, we recommend sticking to softeners or RO units to be sure of good results.
I always find water the most puzzling, complex part of coffee. Question: if most tap water has a lower level of HCO- than Mg+ and Ca+, and when water is boiled all the HCO- ‘pairs up’ with Mg+ and Ca+ (forming limescale), how can there be any HCO- left in the water to reduce its acidity?
i have the same question as you . looking for reply from BH
Hi Will – there’s two things at play here.
The first is that it takes time for limescale to form. If you are boiling a kettle for coffee, only a small amount of limescale will form by the time you brew your coffee, so there will still be plenty of bicarbonate in the water after boiling. You will have to boil your kettle for a long time to drop out all the limescale.
Now, in an espresso machine you effectively have a kettle boiling for a long time – forever, if you don’t switch it off. But in this case, the second factor comes into play – the existing limescale in the boiler starts dissolving into the water as well. There’s an equilibrium point where the amount of limescale being dissolved is the same as the amount being formed – this is the idea behind the Langelier Saturation Index, which you can read more about in The Water Course.
Ah thanks, this makes a lot of sense. So you by holding water at a high temp in a ‘clean’ vessel for a long time you could drop out more bicarb, but filtration obviously becomes a more sensible option.
Hey! I’ve been long puzzled by a simple question: if Mg forms scale just as well as Ca does, why Hendon and M C-D advocate substituting Ca for Mg? Or is Mg somehow “safer” in terms of scaling (given that 95% of sources warn only of Ca, it certainly seems so…)?
Hi, thanks for posting this – it’s a good question.
The short answer is they think it improves flavour. They propose that Mg proportionally extracts more of the desirable flavour compounds. This is based on their own taste testing, and they suggest a mechanism for it via computational chemistry (https://pubs.acs.org/doi/pdf/10.1021/jf501687c) but as far as I know this hasn’t been independently confirmed.
The main reason most sources warn about calcium scaling is that calcium is usually the main component of hardness in drinking water, so magnesium scaling isn’t much of an issue. However, switching calcium for magnesium may also reduce the impact of scale slightly. Magnesium interferes with the crystalline structure of calcium carbonate scale, making it more likely to form aragonite crystals, which are slightly more soluble than calcite crystals formed by pure calcium carbonate. Mg itself forms scales of magnesium carbonate and magnesium hydroxide, but both of these are a bit more soluble than calcium hydroxide at 100C. So it seems possible that having a higher proportion of magnesium is ‘safer’ as well.
Yay, thanks for the answer, it’s really helpful! That’s something I haven’t run into yet.
“…but both of these are a bit more soluble than calcium hydroxide at 100C” – you mean CaCO3 here, right?
I live in the U.S. and it appears from what I can understand from the water quality report from my local water district we have very hard water. So I use bottled water for making coffee. I came across the peak water pitcher. I was wondering if you all have had a chance to check it out.
Hi Timothy. The Peak Water filter reviews really well. Scott Rao was recommending it highly in his recent December newsletter. One comment we have heard a few times is that it is less consistent over time, but this is expected with all ion-exchange systems.
I’ve compared Peak with the Brita Marella filter jug (using Maxtra cartridges). They’re both almost equally efficient in terms of ion exchange (the Brita cartridge was a bit used, so I’ve made a discount for that), but TBH Brita feels much more solid in terms of materials and design. Peak has this low-quality-Chinese-manufactured-plastic feel, unfortunately, and I’m afraid that it won’t last long.