Apart from bicarb and epsom salts…
A few months ago we released an updated version of our water recipes designed to allow you to easily target any hardness and alkalinity level you want, using two simple solutions — without having to do any complex maths. Since writing the post, a few people have written in to ask how to extend this method to use other mineral salts. We’ve written this post to make it as easy as possible to make your water with any mineral salts you would like to experiment with.
The bad news is that in order to do this, you will need to do some basic maths, and a little research into what your mineral contains. The good news is that in this post, we’ve tried to simplify these concepts as much as possible, so that even if you don’t understand the chemistry, you should be able to expand your water repertoire by following these instructions.
What Minerals Can We Use?
The idea with this post is that you can use anything you like, as long as it’s food safe! However, there are a few commonly used minerals to consider.
Hardness
- Calcium Chloride
- Calcium Sulphate
- Magnesium Chloride
- Magnesium Sulphate
- Sodium Bicarbonate
- Potassium Bicarbonate
Both
These minerals are hard to dissolve, so for that reason we don’t recommend using them. If you want to try using these, you can carbonate the water using a sodastream to make them dissolve, but we find there are easier ways to get similar results.
- Calcium Carbonate
- Magnesium Carbonate
Other
- Sodium Chloride
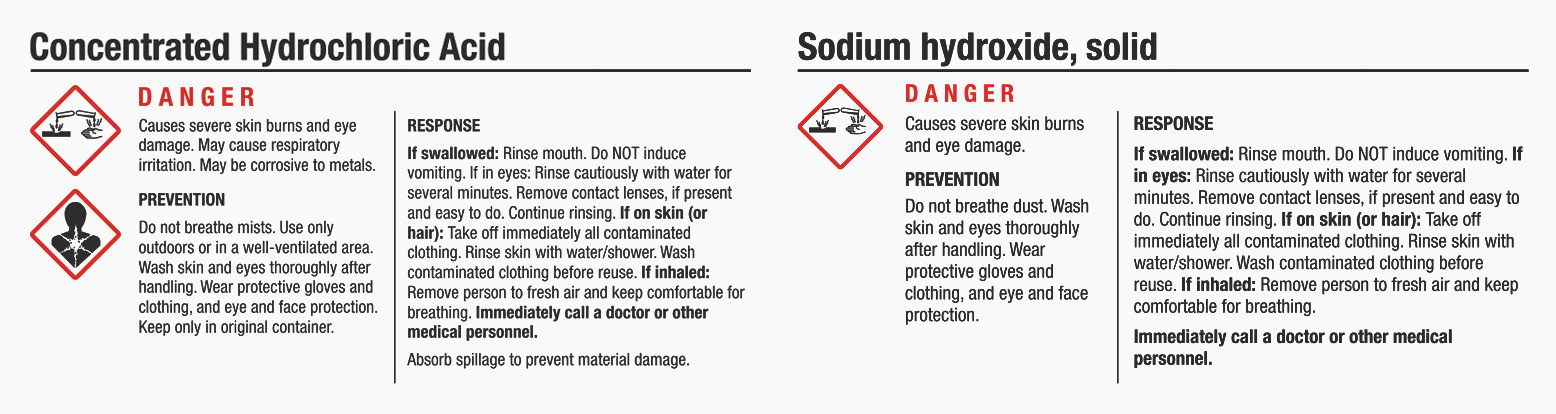
- Sodium Hydroxide
- Hydrochloric Acid
- Sodium Citrate
Calcium and magnesium minerals are responsible for hardness and give your extraction power, and the bicarbonates or carbonates add buffer, to moderate the acidity. These are the major components that control the way the water affects your coffee.
The chloride and sulphate ions don’t seem to have much effect on flavour at the concentrations we typically use, but can be corrosive or impart off flavours at high concentrations.
Sodium may have some effect in extraction, but too much will lend a salty taste to the water. Salt has also been reported to reduce perception of bitterness in small amounts (P. A. S. Breslin & G. K. Beauchamp, 1997).
Sodium Hydroxide and Hydrochloric Acid are used by Chris Hendon and Maxwell Colonna-Dashwood in “Water for Coffee” to control pH. However, because these chemicals are dangerous without careful handling, we don’t recommend using these.
Finally, Sodium Citrate is an ingredient in Third Wave Water, used as a buffer instead of bicarbonates. Using this correctly is a little more complicated and beyond the scope of this article, as it’s an organic molecule rather than a mineral, but we’ve listed it for completeness, should anyone want to experiment with it.
How Much to Use?
It’s maths time! The first step is to work out exactly what the content of your mineral is. Many common minerals exist naturally as a ‘hydrate’, meaning they include water as part of the crystal structure — for example, Epsom Salts are actually Magnesium Sulphate Heptahydrate (MgSO4.7H2O). This should be listed by the manufacturer, but if in doubt, check online for the common form of the mineral and what it should look like.
Once you know what you have, you need to know the molecular weight of the mineral — and rather than revising your high school chemistry, you can just Google the answer: for example, searching “molecular weight of magnesium sulphate” gives me the answer: 120.366 g/mol.
You then need to add the molecular weight of the water in the crystal — 18 g/mol for each water molecule. So for our example with Epsom Salts: 120.366 + (7 × 18) = 246.366 g/mol
Hardness
To work out how much of a calcium or magnesium mineral you need to add to 1L of water to get a certain hardness, just divide that number by 100,000 then multiply by the number of ppm hardness you require.
So here’s how to get 100ppm of hardness, using our example of Epsom Salts: 246.366 ÷ 100000 × 100 = 0.246g added to 1L water.
To work out how much of a bicarbonate you need to add to 1L of water to get a certain alkalinity, just divide the number by 50,000 then multiply by the number of ppm alkalinity you require.
So, using Google we find that the molecular weight of sodium bicarbonate is 84.007 and it contains no extra water (anhydrous). To get 100ppm of alkalinity: 84.007 ÷ 50000 × 100 = 0.168g added to 1L water.
If you do use magnesium carbonate, then for both hardness and alkalinity, just multiply your desired ppm by 0.00084, then use that many grams in a litre of water.
And Then?
That’s pretty much it! To confirm you got the KH and GH you intended, you could use a drop kit to measure the water you’ve made. We don’t know much about how other minerals affect the taste of coffee, so for those minerals (such as table salt), trial and error is the way to go. If you want to tweak pH, the easiest way is to use a pH meter and add your acid or base drop-by-drop until you get the pH you want, but bear in mind that this will also affect the alkalinity.
Weighing such small amounts accurately is hard and needs special scales, which is why we use concentrates. You might find it easier to weigh out ten times as much as you need, then dilute the concentrate 1:10.
Look out for any cloudiness or white deposits at the bottom of your container — this tells you some minerals didn’t dissolve, or reacted together to form something that doesn’t dissolve, which will throw off your calculations.
Lastly, a warning: don’t put remineralised water in your espresso machine unless you’re very sure what you’re doing (in which case, you probably don’t need this post!). If you create super-hard water and scale up your kettle, it’s fairly easy to fix — but descaling an espresso machine is no fun at all.




I just want to confirm the correct weight of Epsom Salts needed for the 1l concentrate. In the Example above the calculations comes to 0.246g to get 100ppm. However, in the post before this it states that 2.45g is needed to obtain 1000ppm. Is the 2.45g right or should it be 2.46g?
Also, according to google the molecular mass of water is 18.01528. Would it be more ideal to use this?
Hi,
Many people use TDS, instead of GH/KH, to describe the water which suit the coffee they brew. Does the number of TDS really means something?
Hi Po-Chen – TDS meters measure the conductivity of the water, which depends on the amount and types of mineral ions in it.
It’s a really quick and easy measurement which is why people like to use it for making quick comparisons or checking their water hasn’t changed – but it doesn’t tell us much about the hardness (GH and KH) of the water, which is what really makes a difference to the coffee flavour.
Have you experimented with adding minerals to brewed coffee? A recent YT video https://www.youtube.com/watch?v=-_8fXToQ1dg sugests that adding minerals to brewed coffee is the same as using mineralized water to brew the coffee. Are you aware of experiments, adding additions to brewed coffee, to improve taste?
Hi! Is it OK to use deionized water in place of distilled or RO water?
Yes it is, but be aware that some deionised water products aren’t made for human consumption. Read the product label carefully.
Good afternoon.
You write: “Finally, Sodium Citrate is an ingredient in Third Wave Water, used as a buffer instead of bicarbonates”.
I studied the composition of preparations for water on the thirdwavewater website
The https://thirdwavewater.com website not Sodium Citrate, but Calcium Citrate is indicated in the composition of preparations for water.
Is there a mistake in the article?
You’re quite right, they use Calcium Citrate. So the Calcium is contributing hardness, while the Citrate adds some buffering capacity. Sodium citrate would work as a buffer in the same way, but without affecting hardness.
Hey guys!
In the TWW packets it indicates sodium chloride as the third ingredient. If calcium citrate is acting to both add hardness and buffer the water, what would the purpose of adding sodium chloride be in combination with the Magnesium Sulfate and Calcium Citrate?
Assuming it’s adds extra buffer, but not sure why the need for so much of it.
We are trying to create our own formula in our coffee shop to remineralize our R/O water and found sodium was extremely low after remineralization in comparison to our tests on TWW.
TWW had extremely high sodium from our testing accounting for a large majority of the TWW Tds.
Is salt actually that helpful in aiding in extraction or regulating total alkalinity?
Thanks so much for any insight!
Hi, thanks for commenting! Sodium does have some effect on extraction according to the SCA – but less so than divalent ions (Calcium and Magnesium). Sodium Chloride doesn’t have any significant buffering capacity though.
A little salt in the water may have other benefits: salt can reduce perception of bitterness and increase perception of sweetness, for example.
Hi there! I’m just wondering if you’d know how to make a calcium concentrate 1000ppm solution? If i use calcium chloride 10%(1gr/10ml) i need to use weight of CaCl in L 2,48gr/l?l(25ml~) to get 1000ppm/l concentrate? I want to mix water with calcium and magnesium concentrate, because i use espresso machine(vbm domobar d2) and heard , that ro water is not good for espresso machine(boiler corrosion and etc)
I want to make three concentrate(Ma, Ca, Bicarb) with 1000ppm/L concentration.
If i will take 2,45gr epsom and disolve it in 997,5ml of water i will take 1000ppm/L GH by Ma
If i will take 1,68 baking soda and disolve it in 998,32ml of water i will take 1000ppm/L KH
How many Calcium chloride 10% i need to add in L of water to get 1000ppm/L GH by Ca? 25ml to 975ml water?
I want to mix after that safety water to espresso machine. I think it will be around 60ppm by Ca, 80ppm by Ma and 40ppm by KH
Hi again! Calcium chloride (anhydrous) molecular weight is 110.98 g/mol, so (following the maths in the post) to get 1000ppm (as CaCO3 equivalent) you need 110.98 ÷ 100000 × 1000 ~= 1.11 g per litre. If you have a solution with 1gr/10ml then you therefore need 11.1ml of your solution, then make it up to 1L with distilled water.
However, bear in mind that adding chloride will actually increase your risk of corrosion. Even though having both calcium and carbonate in your final water will boost your LSI, I can’t say for sure that this water will be safe against corrosion. Ultimately it depends on what your machine is made of: for more on this, check out my AMA on water chemistry where I discuss this in more detail.
Big thank you!
It’s so hard to understand, i never think about corrosion in my espresso machine, and long time used ro water, thinking that mild water great for copper boiler and system…after i read your article i stated worries
Maybe do you know about safety content in bottled water Ca/Mg mg/l and hardness to keep my machine healthy? I will try to find bottled water with this approximately condition
And one more thing
I have 10% with hexahydrate, so in 1000ml concentration 100grams of Cacl2|6•H2O or 100mg in 1ml hexahydrate or 67,1mg in ml dehydrated.
I have no anhydrous, so how much ml with hexa/dehydrate form i have to add
Hi guys,
Are the maths for how much baking soda to add to 1L to get 100ppm exact?
Cause I actually add 0,84gr to 1L to get 1000ppm
Also how can I find the CaCO3 equivalent for Calcium dihydrate? So I can apply the same maths.
Thanks
Hi Cedric – bicarbonate only has half the buffering capacity of carbonate, so you need 2 bicarbonates per CaCO3 equivalent.
As we posted below: “Remember the function of the carbonate/bicarb is to buffer the acidity by removing H+ ions. CaCO3 can neutralise 2 H+ ions (CaCO3 + 2H+ → Ca2+ + CO2 + H2O) while sodium bicarbonate can only neutralise 1 (NaHCO3 + H+ → Na+ + CO2 + H2O)”
Hi, when I saw on BH youtube (DIY Water For Coffee Brewing) it says you put 10.14g of epsom salt in litre of water but on the blog it says put 24.6g (0.246g) of epsom salt add to litre of water which is the right one? Thanks
About adding the magnesium hydrate, I don’t really grasp the simplified mathematics:
Wouldn’t you want to add 0.01 mass-% off magnesium-ions into the solution if you want 100ppm concentration?
0.01% of 1000gr (weight of 1 liter water) would be 0.1 gr of magnesium in solution. The molecular weight of magnesium is approximately 24 gr/mol, 0.1gr / 24 = 0.0041 mols of magnesium and therefore 0.0041 of magnesium-hydrate since each molecule contains one Mg atom. 0.0041*246 = 1gr of Mg-hydrate…..???
Or did my chemistry became mega rusty hahaha
Hi Stijn – we’re aiming for 100ppm of hardness (expressed as CaCO3 equivalents), rather than 100ppm of magnesium ions. Hope that makes it clearer!
I have found Potassium Bicarbonate’s molecular weight to be 100.115. So with the maths included I come up with 2g per liter of distilled water for concentrate. Which should give me the same results for targeting a specific KH or GH as the Sodium Bicarbonate 1.68 in your updated water recipe. I could not find if it was Heptahydrate or Anhydrous and most suppliers do not state.
Do you know if Potassium Bicarbonate is in fact anhydrous ? Otherwise how would I do the maths if it was a Heptahydrate?
Thanks a lot for this article.
Ghazi
Hi Ghazi, Potassium Bicarbonate will be anhydrous. Happy mineralising!
Potassium Bicarbonate KHCO3 is a heptahydrate or at least the one I got. So with the maths included
100.115 + 126 = 226.115 ÷ 50,000 x 100
= 4.5g per litre of concentrate
Hello. Sorry the weight for KHCO3 is
100.115 + 108 = 208.115 ÷ 50,000 x 100
4.2g per litre
Please someone correct me if am wrong
Thanks
Is there any way you could explain how to remineralise water for espresso machines? I’ve been making my own water for filter brewing for quite some time now, but the water for my espresso machine has always been a problem to get right, as filtered tap water isn’t good enough.
How about RO with bypass?
RO would be pointless in espresso since the extraction ratio water:coffee is so low. Where pourover may be 17;1 espresso may be 2:1 so ideally the mineral content could be x8 more concentrated.
It is for this reason I use a ROK manual extraction which is easy to clean and affords pressure profiling
Hey Jonas, You’ll find three detailed lessons on this in chapter 4 of The Water Course which is one of our online courses included in the BH Unlimited subscription.
Why is the alkalinity bit divided by 50000?
I can understand if you are using calcium/magnesium bicarb, but sodium bicarb should only contain one HCO3- group? Or am I being daft
Thanks
You’re not being daft. It’s because with bicarbonates there are twice as many carbonates than sodium/potassium, so dividing by 50,000 is the same as dividing by 100,000 and then multiplying by 2. That part could’ve explained a little better, but it is correct.
Hi Ant – David is correct, on all counts. We skipped over this to avoid a long explanation!
Firstly, why 100,000? It’s the molecular weight of CaCO3 (100), which is our reference molecule, multiplied by 1000 to convert from g to mg/L.
For Bicarb, it’s then half that: 50,000, because you need 2 bicarbonates to make one CaCO3 equivalent.
To understand why two bicarbs is equivalent to one carbonate, remember the function of the carbonate/bicarb is to buffer the acidity by removing H+ ions. CaCO3 can neutralise 2 H+ ions (CaCO3 + 2H+ → Ca2+ + CO2 + H2O) while sodium bicarbonate can only neutralise 1 (NaHCO3 + H+ → Na+ + CO2 + H2O)