Hydrophobic or Hydrophilic?
In chemistry, we encounter many molecules or parts of molecules which form a chain shape. Two of the big players in foam science — the milk fats and the proteins — are chain shaped. As we investigate foam chemistry, we will discuss the tendency of these chain molecules to be hydrophobic and hydrophilic, i.e., repelled by or attracted to water, respectively. With fats, it’s a simple case of heads or tails; the heads are attracted to water. The chains formed by proteins can be quite a bit more complex.
Protein
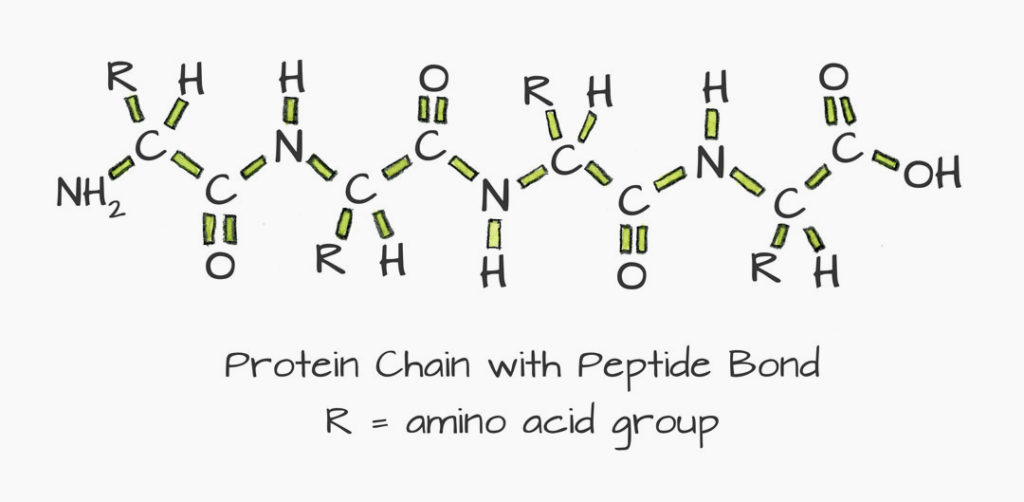
The building blocks of proteins are amino acids. By means of peptide bonds they form a chain structure, as shown in the figure below.
Milk proteins can be arranged into two main groups: the casein family of proteins and the whey protein (also known as serum). Roughly 82% of the protein in milk is casein; the other 18% is whey. Casein contains certain essential amino acids which we humans cannot synthesise in our own bodies. Because casein forms micelles that contain calcium and phosphorus, it is an important nutrient source.
Casein
Casein is a family of a few types of protein — alpha-s1 (α-s1), alpha-s2 (α-s2), beta (ß), and kappa (κ) — each with varying tendencies which help dictate the nutritional value of the milk and its behaviour as a colloid in milk. The small casein particles also happen to scatter light. This phenomenon, called the Tyndall effect, causes milk to appear white, but with a hint of blue if you observe it in a very diluted form.
Note: Due to the Tyndall effect we need to filter samples before measuring the total dissolved solids when performing refractometry with espresso. TDS is the total amount of solids such as minerals, organic, and inorganic matter dissolved in a solution. Refractometry is the process of measuring the TDS of a solution to calculate its strength.
Most caseins are hydrophobic chain proteins.