Innovation and Research
Research by physicist Christopher Hendon and UK cafe owners and roasters Lesley Colonna-Dashwood and Maxwell Colonna-Dashwood found that magnesium ions in water have a high potential to facilitate the extraction of coffee flavour compounds.This means that even if your water has exactly the same hardness and buffer as your roastery, unless you also have the same ratio of minerals that account for your hardness, you can expect quite different flavours in the cup.
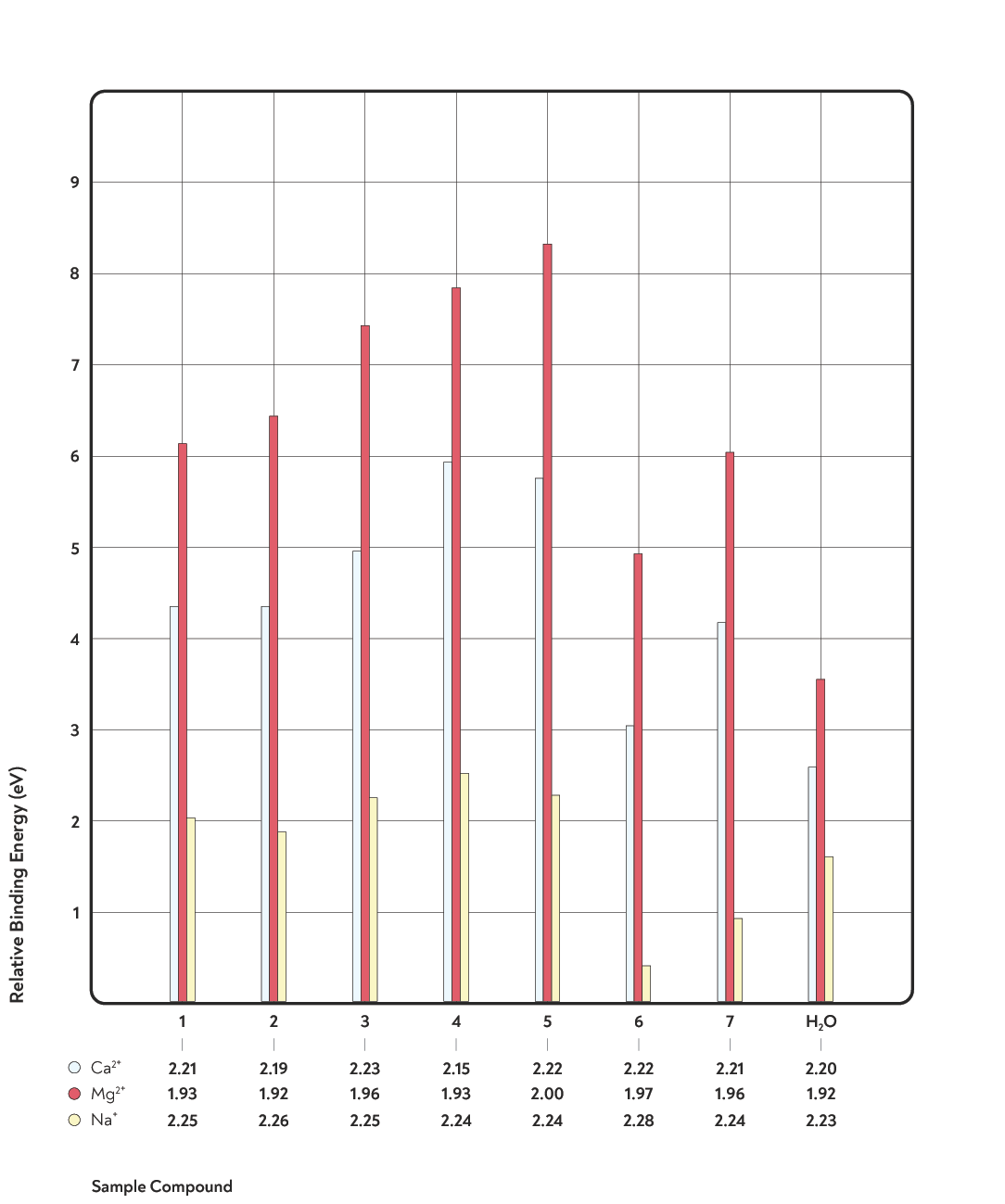
This chart depicts findings from Hendon et al, 2014.
1. Lactic acid 2. Malic acid 3. Citric acid 4. Quinic acid 5. Chlorogenic acids 6. Caffeine 7. Eugenol
In their research paper, Hendon et al. state that ‘Mg2+-rich water is paramount if increasing extraction yield is important.’
They also found that the key coffee flavour components — lactic, malic, and citric acids — could bind more tightly to magnesium ions than to calcium ions. Magnesium ions bound much more strongly than calcium to desirable acids such as citric acid, whereas the difference was smaller for less-desirable acids such as quinic acid.
‘a subtle but important increase in the relative binding energy of 1−3 relative to 4 and 5 to Mg2+ compared with Ca2+ (if only by approximately 5%). This is desirable, suggesting Mg2+-rich water would bind to marginally more of the desirable flavors in coffee, although the macroscopic effects of this would be better probed experimentally. The high binding energies of the undesirable acids, 4 [quinic] and 5 [chlorogenic acids] is less pleasing. Relative to other ions, Mg2+ would significantly increase the extraction of these compounds …’
Binding energy refers to the strength of a chemical bond; a high binding energy describes a bond that is hard to break. Here, Hendon refers to how the dissolved metal ions Ca2+ and Mg2+ bind to flavour compounds in coffee.