A range of sources confirm that the most common way of dealing with wastewater pollution from coffee processing is to dump it into waterways. (J. N. Wintgens, 2004; S. Rattan et al., 2015). When wastewater from coffee processing is treated, the traditional approach is to pour it into a pond around 2 meters deep and leave the water to slowly percolate into the soil. The low pH of pond water can be neutralised by the addition of talc (a clay mineral). The trouble with this simple system is that the rainy season, which usually follows the harvest, can cause ponds to overflow, causing the contaminants to spill into waterways (S. Rattan et al., 2015).
To increase their effectiveness, the ponds can be integrated as the final purification stage in a more complex system such as one suggested by J.C. von Enden et al., (2002) (see below). This system combines the sediment ponds with biogas digestion. Coffee waste contains high amounts of organic substrates — including carbohydrates, proteins, pectins, fibres, and fats — which make it very suitable for conversion into bioproducts such as biodiesel and biogas (Gathuo et al., 1991). However, because some chemicals contained in coffee waste, including caffeine, free phenols, and tannins (polyphenols), are known to be toxic to many life processes, due to public and environmental health concerns, the uptake of potential moneymaking and environmentally friendly schemes such as biogas digestion has been slow.
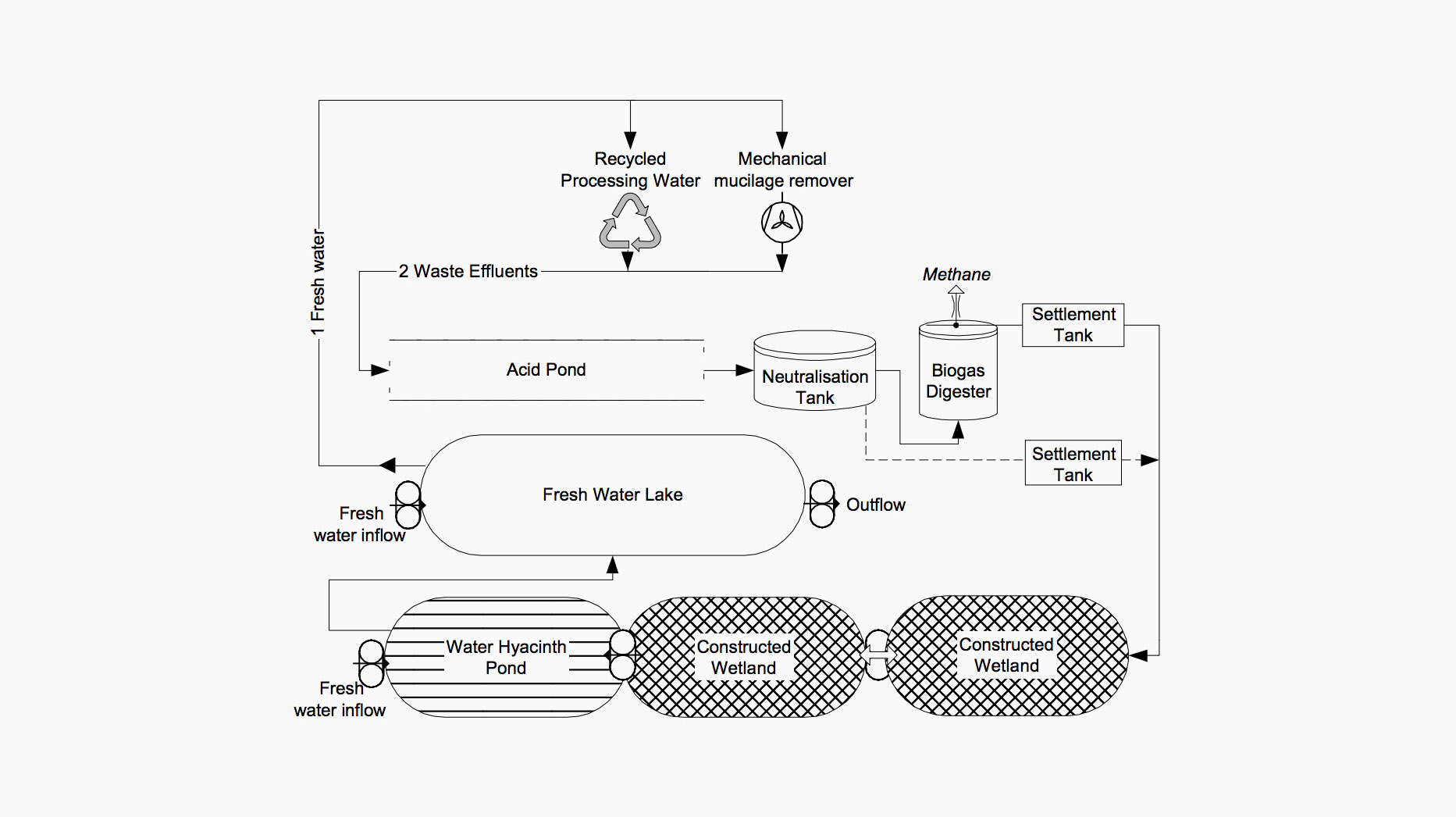 A model for processing spent fermentation water in Vietnam using biogas digestion and reed ponds (J.C. von Enden et al. 2002).
A model for processing spent fermentation water in Vietnam using biogas digestion and reed ponds (J.C. von Enden et al. 2002).
Methods involving biogas digestion look very promising, but they are still regarded as prohibitively expensive. They also require quite precise management of the digester as well as technology to stabilise the temperatures within it. Other technologies, such as the production of biodiesel from waste products, have been explored on a small scale. The large quantities of coffee pulp generated by the industry suggest many potential income streams, such as the production of silage for animal feed, worm farming, vinegar, single-cell protein, biopesticides, and probiotics, but none of these have reached even the scale of a pilot project (Murthy et al., 2012).
The easiest way of utilising the by-products of wet processing, according to J. K. Mburu and P. K. Mwaura (1996), is composting the wastes to organic manure for use as a nutrient source and a soil conditioner. Other potential uses include adding wastes to animal feed mixtures; using them for mushroom production; and utilising them for the extraction of vinegar. Mburu and Mwaura also suggest producing charcoal from coffee husks and using parchment husks in the production of building materials.
The Immediate Solution
It is easier and cheaper to remove solids from a small quantity of heavily concentrated water than it is to remove the same amount of pollutants mixed into many tonnes of water. For this reason, over the last 20 years, many changes have been made to the traditional wet processing systems in places such as Colombia. The less the pulp comes in contact with water, the less polluting potential any water used at later stages will have. Dry pulping and dry fermentation methods don’t require any water. Density separation can be carried out at the dry mill (see Chapter 6) instead of via washing streets.
When weighing the pros and cons of traditional wet processing, it is important to remember that coffees coming from water-intensive washing stations in places such as Ethiopia and Kenya produce some of the most spectacular coffee flavours found anywhere. And there is little doubt that water plays an essential role in achieving these delicate flavour profiles. The goal should therefore be to support this valuable tradition with equitable trading practices — because eco-friendly infrastructure in wet processing requires capital investment that many low-GDP regions, such as Jimma, don’t have.
Wintgens suggests a five-step approach to reducing water pollution in wet processing:
1) Incorporation of semi-dry or fully dry reception tanks instead of using water to wash the cherries into the wet mill.
2) Reduction of the siphons (flotation tanks) to a quarter of their original capacity.
3) Design and implementation of pulpers that operate without water.
4) Mechanical feeding of ripe cherries to the pulpers by means of screw conveyors (feeding in dry cherry).
5) Mechanical conveyance of the pulp by means of conveyor belts and and screw conveyors.
Removing pulp with screw conveyors helps avoid undue water absorption, thereby promoting faster decomposition, facilitating transportation, and minimising noxious odours. You can see a screw conveyor removing the pulp from the pulping machine in this excellent video from San Francisco based green coffee importers, Sweet Marias.
Water Recirculation
When water recirculation is adopted in wet processing, water consumption can be reduced to 8–22 litres of water per kilogram of green coffee — down from 40–80 litres of water per kilogram of green coffee where no recycling is practiced (J. N. Wintgens, 2004, pg. 922). The process is very simple, requiring a series of sedimentation tanks that decant the top layer of each tank into the next tank in the series, as depicted below.
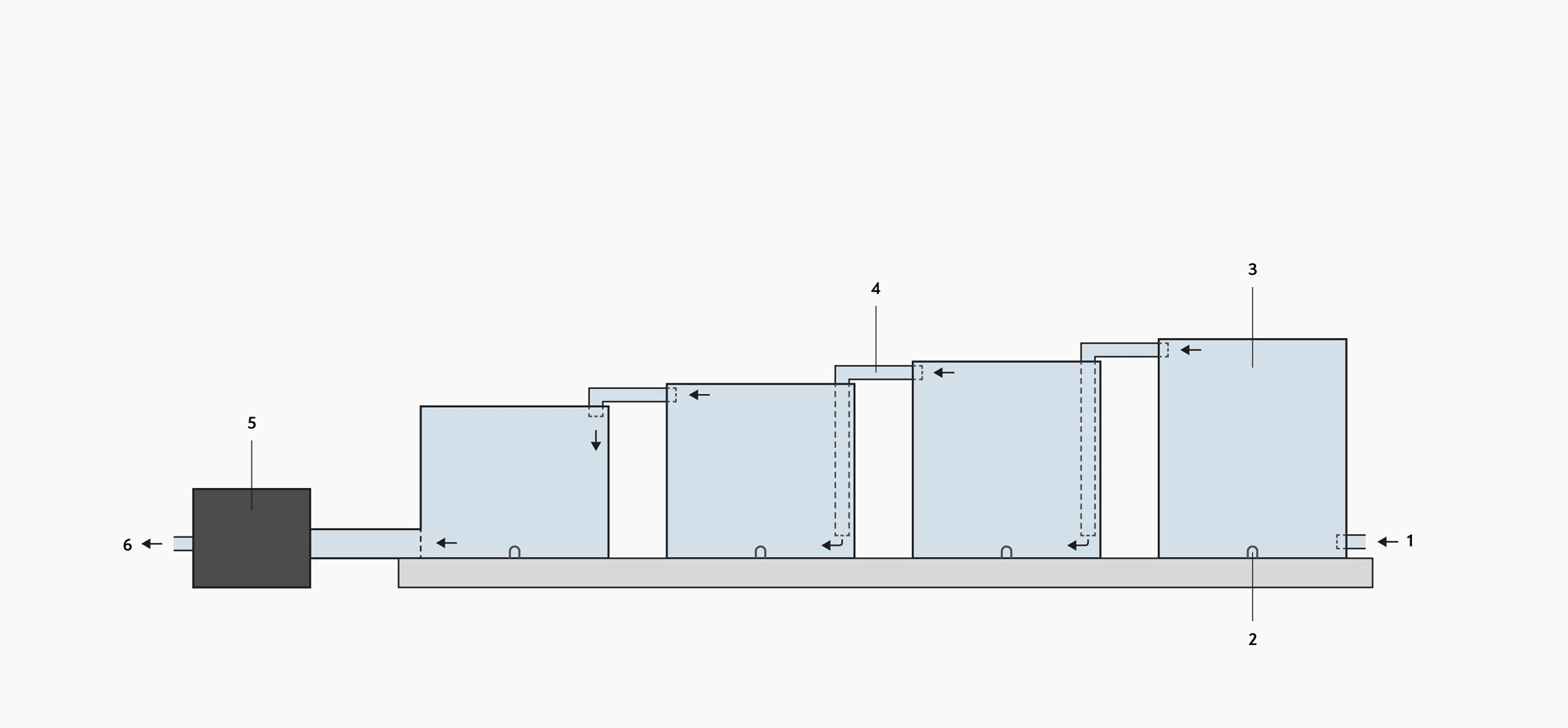 A diagram of a water recirculation system. 1: waste water inlet, 2: drain for cleaning the water tanks, 3: water tanks, 4: pipes, 5: recycling pump, 6: water that returns to the system.
A diagram of a water recirculation system. 1: waste water inlet, 2: drain for cleaning the water tanks, 3: water tanks, 4: pipes, 5: recycling pump, 6: water that returns to the system.
The diagram above shows how sediment is removed from water that can be reused in flotation tanks and pulping. It is not recommended to use recycled water for wet fermentations. The longer the recycled water is reused, the more likely it is that harmful organisms and chemicals, such as ammonia, could develop in it. Wintgens recommends that recycled water should be used for a maximum of 48 hours to avoid contamination of the coffee parchment (J. N. Wintgens, 2004, pg. 653).